Simplify your FDA submittals while shortening your time to market with Intertek's FDA ASCA Program
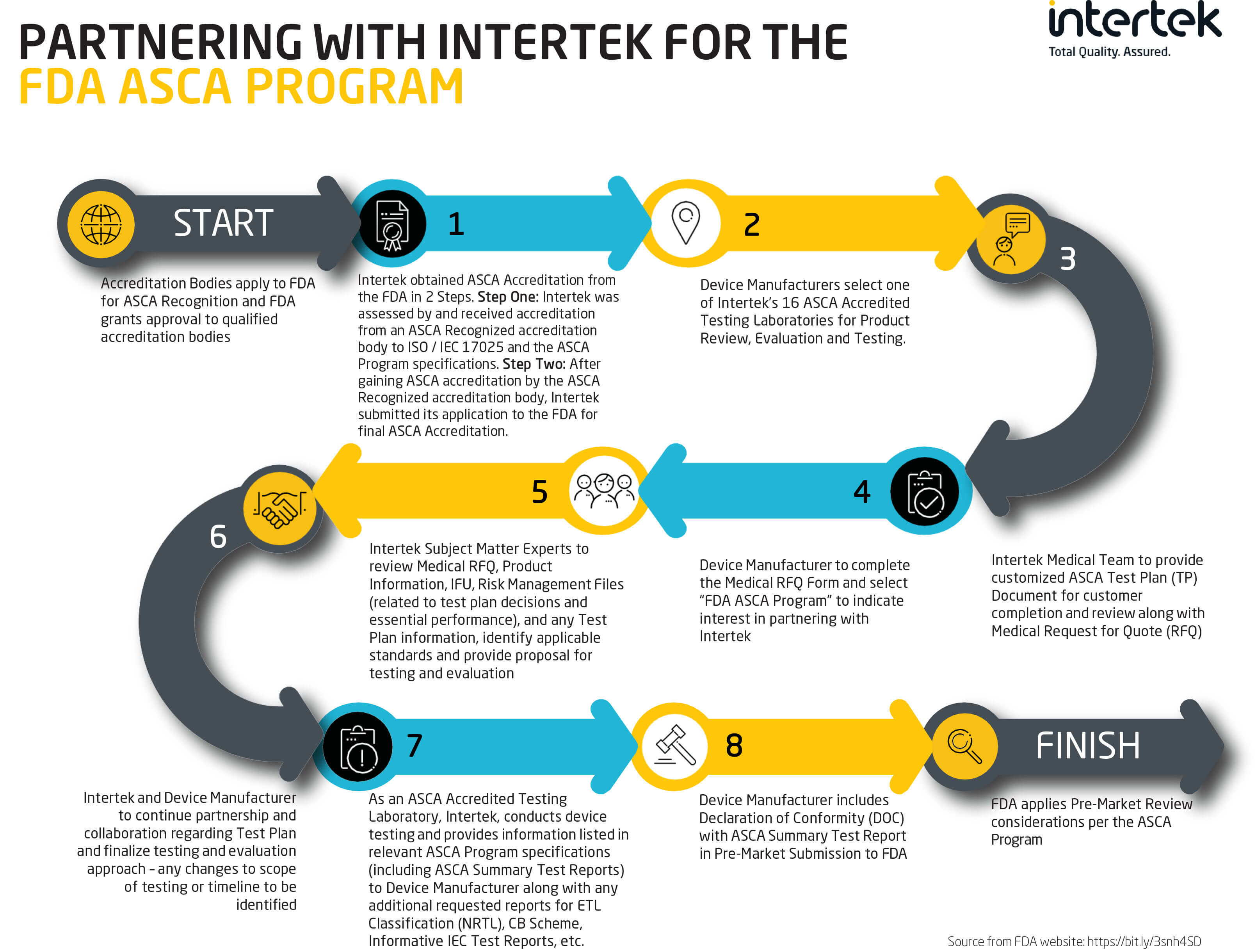
The U.S. Food & Drug Administration (FDA) launched a new program called “Accreditation Scheme for Conformity Assessment”, or “ASCA” in September of 2020. It’s a voluntary program that was developed to help increase confidence in medical device testing, therefore decreasing the need for the FDA to request additional information from manufacturers during premarket submissions. In September 2023, the FDA transitioned its pilot status into a permanent program.
FDA ASCA Program Q&A
November 2023 Update w/ FDA - Webinar
Under the program, testing laboratories apply to the FDA for ASCA Accreditation. Once approved, device manufacturers will be able to contact ASCA-accredited laboratories to begin testing. Once testing is completed, the laboratory will provide results back to the manufacturer, who can then finally make their premarket submission to the FDA.
Intertek now has 16 laboratories officially listed on the “ASCA-Accredited Testing Laboratories” site, including 9 across the U.S., 3 in Japan, 2 in China, 1 in Italy, and 1 in Sweden. No other medical device testing laboratory offers the combination of scope, reach, expertise, accreditations, and commitment to customer success that Intertek provides its customers every day.
There are two primary standards covering Basic safety and essential performance that are included in the FDA ASCA Program:
|
Standard |
Standard Title |
|
ANSI/AAMI 60601-1 |
Medical electrical equipment - Part 1: General requirements for basic safety and essential performance (along with the FDA-recognized collateral and particular standards in the IEC/ISO 60601/80601 family) |
|
IEC 61010-1 |
Safety requirements for electrical equipment for measurement, control, and laboratory use – Part 1: General requirements (along with the FDA-recognized particular standards in the IEC 61010 family) |
A number of Biocompatibility standards will also be included in the FDA ASCA program. Please view the ASCA page on the FDA’s website to learn more.
Knowledge Center
Evolution of the Home Healthcare Market and Medical Device Compliance
White Paper | Webinar Recording
Ventilator Production: Introductory Guide to Regulatory Requirements
Critical Care Medical Devices: First-In-Queue Priority
Product Listing & Marking Strategies White Paper
IEC 60601-1-2 Edition 4 White Paper
Updated FCC Approval Process Webinar
Extractables and Leachables Studies Fact Sheet
Five Steps to Medical Device Commercialization in the United States White Paper
Making Green Profitable: Using IEC 60601-1-9 as a Competitive Advantage White Paper
Related Links
- ETL Mark The Industry's Fastest Certification Program
- Search and Buy Medical Device Standards
- Reese's Law – ANSI/UL 4200A-2023
- My Test Central
- Directories
- Certification Marks
- Global Research & Certification
- Medical Podcast - Compliance with Clarissa
- Satellite Data Acceptance Program
- Planning for Quality through Disruptions
- Intertek's world-class team of Medical Experts
- Intertek Protek - The world’s first industry-agnostic, end-to-end health, safety and wellbeing assurance program
Total Quality Assurance during Novel Coronavirus
Intertek provides mission-critical quality assurance solutions to ensure production and operations continue to function smoothly in rapidly changing situations. Intertek’s first priority is always health and safety while delivering outstanding service to our customers.