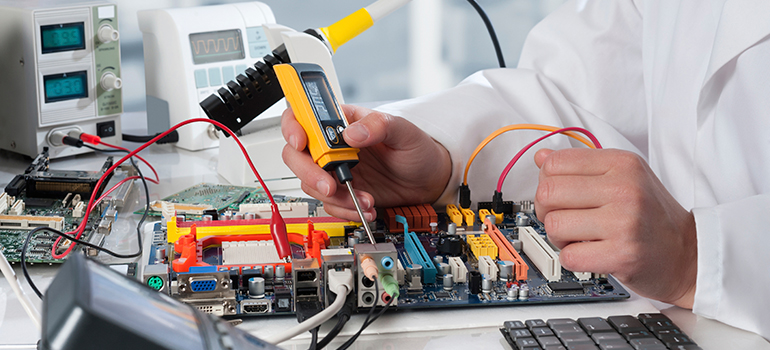
Meet Intertek at the Nasal Drug Delivery Conference

Intertek are attending and exhibiting at Nasal Drug Delivery in London, UK April 11-12 2019. Join us to discuss our nasal product development services for both new and generic projects
The Nasal Drug Delivery Conference 2019, will focus on current industry challenges including optimizing formulation development, novel approaches to device screening and how to approach in-vitro only bioequivalence studies. Arrange a meeting during this 2 day seminar in London (April 11-12).
Our nasal drug development scientists provide method development and validation and testing services to help you optimise the performance of your nasal drug products. Delivered from our Good Manufacturing Compliant (GMP) laboratories, we provide product characterisation, formulation development and analysis, device screening and clinical manufacturing. We can support you from early-phase development to CMC testing, and from clinical manufacturing through to finished product release testing. We routinely provide in-vitro bioequivalence studies (IV-BE) for generic nasal products, including full GMP statistical analysis and dossier generation.
- Integrated formulation development and scale-up
- Device screening
- Product characterisation
- In-vitro bioequivalence
- Stability studies
- Clinical supply manufacture
RESOURCES:
On Demand Webinar: Advanced analytical techniques for testing generic OINDPs
On Demand Webinar: Stability Programs for Orally Inhaled or Nasal Drug Products
Article Download: Overcoming the Challenges to Inhaled Biologics
Brochure Download: Intertek Inhaled Product Development Brochure
Our GMP Center of Excellence for orally inhaled and nasal drug product development is located in Melbourn, near Cambridge, UK. It is equipped with the latest nasal and inhalation drug product testing instrumentation and our scientists apply our 25 years of experience in analytical and formulation support to the development of nasal solutions, suspensions and dry powders, providing the critical performance and quality testing that you need for successful product development. Bringing quality and safety to life, we offer Total Quality Assurance expertise to help you to meet and exceed quality, safety and regulatory standards.
ON DEMAND WEBINARS
Repurposing Products for Inhaled Delivery - Rapid Response Strategies
Formulation and Manufacturing Approaches for Nasal Drug Products
ARTICLE DOWNLOADS
Repurposing Vaccines for Intranasal Development
In Vitro Bioequivalence for Pulmonary and Nasal Delivery
Nebulised Drug Development Considerations
Assessment of Foreign Particulate Matter in DPI Formulations
NEW! Discover our Audit Live Tool for direct access to our scheduled audits
EVENT: 2nd Annual Inhaled and Nasal Biologics/DNA Forum - REGISTRATION IS LIVE
VIDEO:EXTRACTABLES/LEACHABLES LAB TOUR - Request access
WORKSHOP: How to Build an Inhaler (RDD 2023)
PRESENTATION:E&L Strategies (3rd Annual Extractables & Leachables Conference)
TALK: Medical Device Extractables & Leachables Studies
TALK: ID and sensitivity issues during E&L studies
ARTICLE: Glycosylation Analytical Approaches for Antibody Therapeutics
APP NOTE: Rapid Determination of Low/Trace Level Benzene in Pharmaceutical Excipients and Finished Products
ARTICLE: Importance of Aligning a Pharma Audit Scope with the Correct Standard
TALK: mRNA Analytics: Capping Efficiency, Sequencing, Poly-A Tail, dsRNA
Intertek Melbourn
GMP Pharmaceutical Analysis and Formulation Expertise
Intertek Melbourn
Saxon Way, Melbourn
Herts, SG8 6DN
UK
Recent News:
Laboratory Expansion for Specialist OINDP Development for Biologics